Gene Expression in Carnation Cells Induced by Fusarium oxysporum f.sp. dianthi
| Received 20 Mar, 2025 |
Accepted 28 Jun, 2025 |
Published 30 Jun, 2025 |
Background and Objective: Carnations (Dianthus caryophyllus) are a major export in global floriculture, but their production is threatened by fusariosis, caused by Fusarium oxysporum f.sp. dianthi (FOX), a pathogen responsible for vascular wilt and significant yield losses. This study aimed to investigate the gene expression patterns in carnation cells in response to FOX infection. Materials and Methods: Carnation varieties with known susceptibility or resistance to FOX were selected. Leaf explants were cultured in vitro on MS media with 2,4-D to produce undifferentiated cells. Elicitation was performed by exposing these cells to FOX conidia. The mRNA was extracted using the Trizol® method and purified with the NucleoSpin mRNA® kit. De novo transcriptome sequencing was carried out using the TruSeq Stranded mRNA LT Sample Prep Kit and the NovoSeq 6000 S4 Reagent Kit by Macrogen (Korea). Unigenes were identified using the CD-HIT-EST program, annotated against public databases, and analyzed for predicted Open Reading Frames (ORFs). Results: A total of 85,669 unigenes were identified, of which 32,101 (37.4%) were successfully annotated. Differential expression analysis revealed significant variations in gene expression between elicited and non-elicited cells in resistant and susceptible varieties. Key genes such as Phenylalanine ammonia-lyase, Anthocyanin 5-O-glucosyltransferase, Beta-1,3-glucanase, and Chitinase 4-like glycosyl hydrolase showed altered expression, along with 24 other genes potentially involved in plant defense mechanisms. Conclusion: The findings indicate that FOX infection induces a multigene, multivariate response in carnation cells. Gene expression patterns differ significantly between resistant and susceptible varieties, highlighting the complex genetic basis of resistance. These insights can contribute to more efficient breeding strategies for developing FOX-resistant carnation cultivars.
INTRODUCTION
Carnations are the second most exported flower globally and are produced by more than thirty countries. The demand for carnations is predominantly concentrated in the USA, Europe, and Asia, accounting for approximately 90% of the market share. The global trade of this flower is estimated to be around $31.95 billion in 2023, and for some countries, it represents a significant source of income1. Colombia holds the distinction of being the second-largest flower exporter and the leading carnation producer on a worldwide scale2. However, the average yield experienced a decline, dropping from 31 ton/ha in 2019 to 28.4 ton/ha in 2022. Diseases affecting plants’ crops are the primary factors contributing to production losses2.
Members of the Fusarium genus are filamentous ascomycetes characterized by a well-developed septate mycelium and distinctive conidiophores, along with resistance structures such as chlamydospores that can persist in soil for decades3. Fusarium oxysporum, a cosmopolitan pathogen, has been responsible for losses of up to 47% in tomato crops4 F. oxysporum f.sp. Cubense Race 4, renamed Fusarium odoratissimum, has adversely affected more than 80% of banana crops. In the case of carnations, losses of up to 40% have been attributed to the pathogen Fusarium oxysporum f. sp. dianthi (FOX) Race 25.
Fusariosis in carnations is a disease that leads to losses at all stages of the crop and during all phonological phases of the plant3. Currently, the most effective method for disease prevention is the utilization of resistant plants, which are developed through breeding programs at a significant cost. The primary molecular techniques employed to identify resistant carnation plants include molecular markers. However, the results obtained through these identification techniques do not necessarily lead to the production of resistant plants within the context of long-term breeding programs. Previously, three pairs of genes responsible for resistance to FOX were identified through crossbreeding techniques in the field in F1 and F2 hybrid carnation lines3.
In 2014, Yagi and collaborators5 published the complete genome sequence of carnation and identified 1050 tRNA, 13 rRNA, 92 snoRNA, and 143 miRNA sequences. Additionally, they reported the presence of 43,266 protein-encoding genes. Li et al.6 reported the complete carnation chloroplast genome, which included 112 unigenes, including 78 protein-encoding genes, and described the characterization of a family of heat shock transcription factors (Hsfs), a type of important transcription factor class, which plays significant roles in protecting plants from damage caused by abiotic stress. In the BMC Genomics database, Tanase et al.7 reported transcriptome analysis of carnations based on next-generation sequencing technology, revealing 262,896 singlets.
This study provides evidence of significant modifications in the metabolism of carnation cells cultured in vitro and elicited with FOX in a dual culture system. These modifications represent the activation of the plant’s resistance mechanisms. This study examines the gene expression changes in carnation cells induced by Fusarium oxysporum f. sp. dianthi, aiming to understand the molecular responses of the plant to the pathogen.
MATERIALS AND METHODS
Study area: The present work was carried out between the years 2019 to 2021. And was confirmed in the posterior works into 2023 in the vegetal biotechnology laboratory in the Militar Nueva Granada University.
Biological material: Carnation plants produced within a breeding program at the UMNG in Cajicá, a municipality in the department of Cundinamarca, Colombia, were utilized in this study. Plant code SH2, which is susceptible to FOX, was derived from non-commercial carnation varieties. Plant code 395, resistant to FOX, was obtained through a cross between the commercial variety Kaly susceptible (S) and Candy resistant (R), while plant code F1B1 and 6455, (R) and (S) respectively, were obtained in an F2 cross from the previous cross between Kaly and Candy. The seeds that produced the hybrid plants used in this study were germinated in vitro culture using MS media8, with 35 g/L of sucrose and 3 g/L of Phytagel®. Leaf explants from the evaluated plants of each code were placed on MS media supplemented with 2-4-D hormone (1 ppm)3 under in vitro conditions to generate undifferentiated or non-native cells. The cells obtained were re-cultured in the same media no more than four times before being used for elicitation assays.
The FOX fungi, collected from commercial carnation farms, were isolated from non-healthy plants exhibiting symptoms of vascular wilt. The plant surfaces were disinfected with 5% NaClO, and 5 mm fragments of symptomatic stems were cultured on PDA media. Once the fungus appeared, a monosporic culture was established in the same media. Finally, the fungi were cultured in Czapek Dox® liquid medium to obtain conidia.
Dual culture: Three grams of the cells from the hybrid lines were cultured in a Petri dish containing MS media supplemented with 2,4-D for 7 days. Subsequently, 10 μL of a 1×106 FOX conidia/mL solution was applied to the edge of the Petri dish where the carnation cells were growing. This dual culture was sustained for 7 days, with strict precautions taken to prevent contact between the FOX mycelia and the plant cells9.
RNA extraction: Elicited cells (E) with FOX from hybrids F1B2 and 395 (R), as well as SH2 and 6455 (S), were compared with non-elicited cells (NE) from hybrids 395 and 6455. The isolation and purification of mRNA were conducted using the Trizol® method with the NucleoSpin mRNA® kit from Macherey Nagel. The concentration of the extracted mRNA was determined using the Qubit RNA BR Assay Kit from Thermo Fisher Scientific, and the integrity was assessed using a 2100 Agilent Bioanalyzer from Agilent Technologies.
Transcriptome: De novo transcriptome sequencing was performed by Macrogen (Korea) using the TruSeq Stranded mRNA LT Sample Prep Kit and the NovoSeq 6000 S4 Reagent Kit. Each sample was subjected to two reads. De novo transcriptome assembly was carried out to reconstruct transcript sequences without relying on a reference genome. The identified unigenes, obtained using the CD-HIT-EST program, were compared against public databases, and predicted open reading frames (ORFs) were analyzed to identify protein-coding regions. Read trimming for all the samples was combined into a single file to create the reference transcriptome, composed of assembled fragments known as contigs.
RESULTS
In preliminary in vitro experiments involving dual cultures of cells and micro-plants elicited with FOX; as well as, ex vitro assays conducted in a greenhouse with pots-plants similarly elicited9, we have established the following observations: Carnation variety SH2 exhibits the highest susceptibility to FOX, while 6455 displays a lower or partial susceptibility to the pathogen. Variety 395 demonstrates the highest level of resistance to the fungus, whereas F1B2 is a carnation variety exhibiting lower resistance or partial resistance.
The variety 6455(S)E exhibited the highest total RNA concentration, measuring 0,13 μg RNA/μg cells, as determined using the ARN-Nano 6000 bioanalysis chip. This result was further validated using the Qubit technique. In contrast, variety 395(R)E displayed the lowest total RNA concentration, at 0,0038 μg RNA/μg cells, and this outcome was replicated in the 6455(S)NE and 395(R)NE varieties. In the NE state, the varieties 6455(S) and 395(R) exhibited similar differences in total RNA concentration, with readings of 0,032 and 0,0031 μg RNA/μg cells, respectively. These findings suggest that total RNA production may be a varietal characteristic independent of the physiological state in NE cells. On the other hand, FOX-elicited cells increased RNA production in resistant and susceptible cells compared with the non-elicited cells in some cases.
Various databases were employed, yielding different results. The read-annotated ratio was 37.4%, with 32,101 sequences recognized. The overall mapping ratio for all sample origins, whether resistant or susceptible to FOX, ranged between 54 and 61%, with no discernible differences detected in the read-mapping ratio. Notably, the total number of trimmed reads exhibited significant variation, with the highest value recorded for 395(R)NE at 26,789,840 reads and the lowest for 395(R)E at 11,559,688 reads. The de novo assembly of transcript contigs yielded 93,246 Trinity transcripts in general in all de varieties. Only the longest isoform per gene with a GC percentage of 40.5 was selected, with an average contig length of 700 bp. For the assembled genes, Trinity transcripts were filtered and clustered into non-redundant transcripts, which were subsequently used for ORF prediction and annotation against databases. A total of 85,669 clustered contigs or unigenes were identified, with a GC percentage of 40.3 and an average length of 726.8 bp. The overall mapping ratio for each sample indicates that the number of mapped reads ranged from 54.5-61.04% for all processed reads.
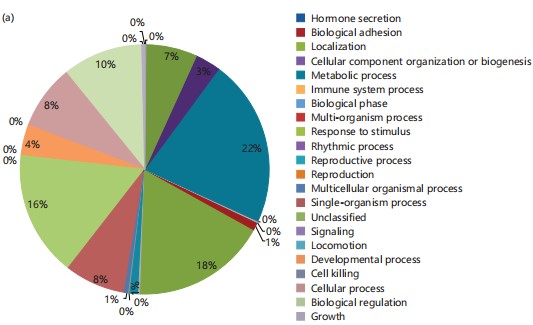
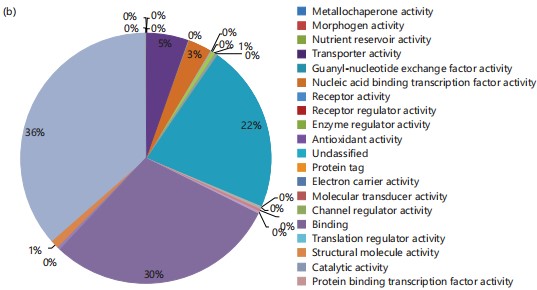
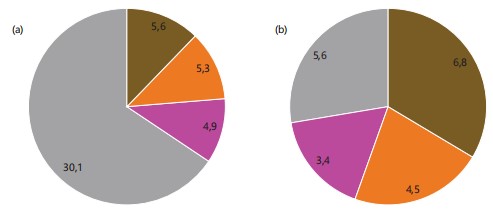
In the context of biological processes (Fig. 1a), 22% of the identified genes are associated with metabolic processes, 8% are linked to immune system processes, and 0.1% each contribute to signaling and cell killing, all of which play roles in pathogen response. Regarding molecular function (Fig. 1b), 3% of the genes are involved in transcription factors binding to nucleic acids, a crucial aspect of pathogen response.
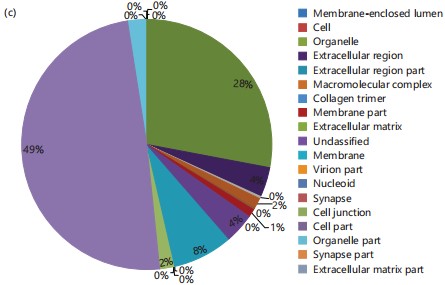
Cellular component and protein identification: Regarding the cellular component, 49% corresponds to unidentified cellular components, 28% represents organelle proteins, 4% relates to protein sequences associated with extracellular components, and 1% involves genes participating in membrane proteins (Fig. 1c). The utilization of the protein identification program EggNOG-mapper allowed the identification of orthologous groups, with one of the most significant groups comprising sequences categorized under defense mechanisms, accounting for 2% of all orthologous groups, and less than 0.1% associated with signaling mechanisms.
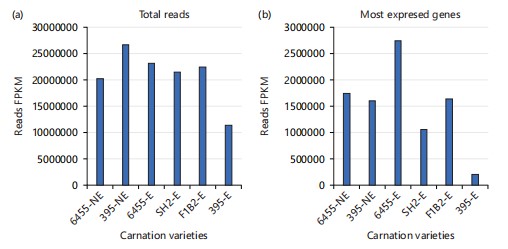
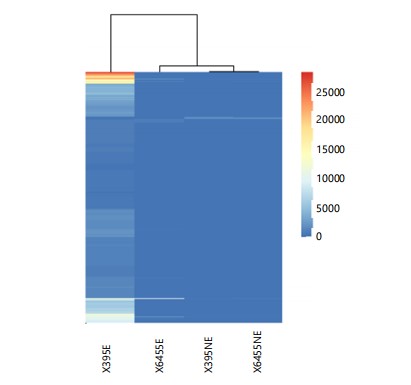
In Fig. 2a, the graphs display the total reads for each carnation variety, whether elicited or not.
A remarkable observation occurs when examining the expression values (FPKM) of the most highly expressed genes (Fig. 2b).
In this context, the susceptible variety 6455(S)E exhibits the highest expression values for its most highly expressed genes, while the resistant variety 395(R)E demonstrates the lowest values, with a significant difference. However, a different pattern is observed when comparing the results for SH2(S)E and F1B2(R)E here, the values are inverted, though the difference is not as pronounced as in the 6455(S)E/395(R)E case. Remarkably, the most highly expressed genes in the resistant and susceptible non-elicited varieties display similar values. It is also noteworthy that when susceptible varieties 6455(S) and SH2(S) are not elicited, they exhibit substantial differences in the number of expressed genes (Fig. 2a). A similar pattern is observed between the two resistant elicited varieties.
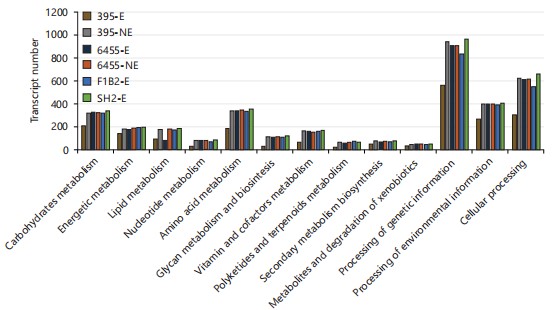
Metabolic behavior: The number of transcripts associated with metabolic pathways in various elicited and non-elicited varieties showed minimal variation. Across all cases examined (thirteen metabolic routes reviewed in Fig. 3), variety 395(R)E consistently exhibited the lowest number of transcripts produced, while SH2(S)E had the highest number of transcripts. The most substantial variation between non-elicited and elicited states was observed in the 395(R) variety, in contrast to the 6455(S) variety, which displayed the least variation in transcript numbers across different metabolic pathways. Notably, the most affected metabolic pathway in terms of transcript number variation among different carnation varieties was the processing of genetic information and lipid metabolism.
|
|
|
Gene behavior: Figure 4a shows that Aspergillus niger and Fusarium spp. caused the most significant reduction in seed germination, while the control group recorded the highest germination percentage, indicating that these pathogens severely inhibit seed viability. Figure 4b reveals that the mean seedling length was also adversely affected by the same pathogens, with infected seedlings being notably shorter than those in the control, suggesting that these fungi impair early growth. Furthermore, Fig. 5 demonstrates that Aspergillus niger and Fusarium spp. led to the highest disease incidence among the tested pathogens, while no infection was observed in the control. Together, these figures underscore the detrimental effects of seed-borne fungal pathogens on germination, seedling vigor, and overall plant health.
Twenty-seven genes displaying a high relationship with expression level variations in response to FOX elicitation across diverse carnation varieties cells were selected (Table 1). The predominant gene influencing the change in expression level is Phenylalanine ammonia-lyase, known for its contribution to plant defense.
|
|
Additionally, proteins such as Beta-1,3-glucanase, Anthocyanin 5-O-glucosyltransferase, and Chitinase 4-like glycosyl hydrolase exhibit increased expression during elicitation. Other genes, including α and β Amylase, Salicylic acid-mediated signaling pathway, Fructokinase, and Ethylene receptor gene, are likely participants in the resistance mechanisms of carnation varieties with resistance. Moreover, proteins like Alcohol dehydrogenase I, Glyceraldehyde-3-phosphate dehydrogenase, Xyloglucan endotransglucosylase/hydrolase, Ribulose bisphosphate carboxylase/oxygenase, and a highly expressed Tonoplast intrinsic protein gamma are all overexpressed in one of the two resistant varieties, though not consistently in both.
| Table 1: | List of most representative genes potentially signifying an essential role in carnation response to Fusarium oxysporum f.sp. dianthi | |||
| No | SJA | Gene full name |
| 1 | ADH | Alcohol dehydrogenase I (18671) |
| 2 | ADC | Arginine decarboxylase (38063) |
| 3 | GTI | Flavonol 3-O-glucosyltransferase (56048) |
| 4 | EIL3 | Ethylene Insensitive Like 3 (54048) |
| 5 | HCBT | Anthranilate N-benzoyltransferase (4247) |
| 6 | ETR1 | Ethylene receptor gene (2767) |
| 7 | PAL | Phenylalanine ammonia-lyase (13751) |
| 8 | UGT | Anthocyanin 5-O-glucosyltransferase (11039) |
| 9 | GLU | Beta-1,3-glucanase (10925) |
| 10 | CTS | Chitinase 4-like glycosyl hydrolase (25028) |
| 11 | BGLU | β-Glycosidase glycoside hydrolase (6768) |
| 12 | AEP | Aldose 1-epimerase (28123) |
| 13 | TLP | Thaumatin like protein (3959) |
| 14 | ABAM | α and β Amylase (105) |
| 15 | PER | Homologous to peroxidases (2878) |
| 16 | CAT | Catalase (31671) |
| 17 | MDH | Malate dehydrogenase (18362) |
| 18 | MAPK | Jasmonic acid-mediated signaling pathway (4214) |
| 19 | PGIP | Polygalacturonase inhibiting protein (31653) |
| 20 | RDC | Salicylic acid-mediated signaling pathway (13044) |
| 21 | RLK | Receptor-like kinase (9311) |
| 22 | RuBP | Ribulose bisphosphate carboxylase/oxygenase (48496) |
| 23 | FRK | Fructokinase (55984) |
| 24 | RPL | Ribosomal protein L7 (22284) |
| 25 | XTH | Xyloglucan endotransglucosylase/hydrolase (17615) |
| 26 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase (8698) |
| 27 | TIP | Tonoplast intrinsic protein gamma (50626) |
| The number in parentheses preceding the gene’s full name corresponds to the ID in the Kazusa Database and SJA: Standard Journal Abbreviation | ||
DISCUSSION
Carnation fusariosis stands out as a primary contributor to crop losses, underscoring the significant importance of the carnation flower in the global floriculture industry. Identifying the genes involved in plant resistance mechanisms holds promise for the rapid selection of resistant varieties within breeding programs. Currently, the genetic mechanisms responsible for resistance against the FOX parasite in carnations remain unknown. Therefore, it is imperative to investigate the genes involved in these resistance mechanisms. Transcriptome analysis of cells during elicitation with the parasite offers a direct approach to identifying genes engaged in the response, utilizing a simplified model. In this study, cells from four carnation varieties underwent analysis in two distinct experiments: One involving interaction with the FOX parasite in a dual culture or elicited and the other without the presence of the parasite or not elicited. Of the varieties utilized, two displayed resistances 395 and F1B2, while two were susceptible to FOX SH2 and 6455. The resistance exhibited by all four varieties was consistent across cell and micro-plant cultures under in vitro conditions, as well as in greenhouse assays; this consistency in the response to FOX in each variety was akin to what has been demonstrated in previous studies of Soto-Sedano et al.3 and Filgueira-Duarte et al.10.
The different carnation varieties exhibited variations in gene expression numbers, ranging from a maximum of 26 million reads to a minimum of 11 million in the transcriptome. This variability is attributed to gene architecture and structural properties of promoters11. More than 4% of the expressed genes within the biological process category were associated with plant immune system processes, accounting for 299 genes that likely participate in responding to the parasite’s presence, in the same form as has been demonstrated in different plant parasite model12. Among the varieties, the most significant variation in total expression level was observed in 395(R)NE. Conversely, the same variety, when elicited with FOX 395(R)E, exhibited a lower level of total expression. The susceptible variety, 6455E, displayed the highest expression value among the elicited varieties. Interestingly, the most resistant variety, 395, exhibited the lowest values of expressed genes during elicitation. Furthermore, 395E demonstrated the highest percentage variation in gene expression. In other words, 395 exhibited the most substantial difference in the expression of genes with the highest expression levels among all varieties, aligning with its superior resistance in parasite assays compared to F1B2. These expression levels align with findings in other plant studies13.
The analysis of the expression levels of various genes related to or responsible for the plant’s response to the parasite’s presence revealed significant variations during interaction (elicitation) with FOX in vitro assays involving cells from different carnation varieties with distinct responses to FOX, either resistant or susceptible. This variation in expression levels and the number of expressed genes during the parasite interaction represents an adaptive mechanism that enables the plant to employ different strategies against the genetic variation of the parasite. Two proteins, Ribulose bisphosphate carboxylase/oxygenase and Alcohol dehydrogenase I, exhibited the most pronounced differences in expression when comparing the resistant F1B2(R)NE variety with the two susceptible varieties, SH2(S)NE and 6455(S)NE. However, in 395(R)E, these two proteins did not show significant increases compared to F1B2(R)E. It is suggested that cellular respiration intensifies in response to the parasite attack, although this might not be as critical in the broader resistance mechanism14. Additionally, proteins like Glyceraldehyde-3-phosphate dehydrogenase, Jasmonic acid-mediated signaling pathway, β-Glycosidase glycoside hydrolase, and Arginine decarboxylase exhibited considerable increases in expression in 395(R)E compared to their expression in the susceptible SH2-E and 6455-E varieties. Glyceraldehyde-3-phosphate dehydrogenase is a ubiquitous protein with pivotal roles in plant metabolism and stress responses, although the exact mechanism of its function in plant stress resistance remains unclear15. These results indicate that each variety presents distinct genetic expression profiles independently to respond to the presence of the parasite.
Phenylalanine ammonia-lyase (PAL), a protein acknowledged for its role in plant defense mechanisms, is responsible for synthesizing phenylalanine via the shikimic acid pathway16. The L-Phenylalanine, derived from the shikimic acid pathway, is directly utilized for protein synthesis in plants or metabolized through the phenylpropanoid pathway17. In a study by Ardila et al.18 on carnations, a tolerant variety exhibited the induction of the PAL gene, associated with plant resistance, within 48 hrs post-inoculation in roots. However, the authors argued that the levels of PAL mRNA were not increased.
In this study, a threefold increase in PAL expression was observed in variety 395R compared to the partially resistant variety F2BR, with a twentyfold greater increase compared to the susceptible varieties SH2S and 6455S. Another set of proteins with a significant role in the expression of resistant carnation varieties includes peroxidases. In the cases of 395R and F1B2R, their expression increased by more than 25% compared to the susceptible varieties. Ardila et al.18 had previously demonstrated the involvement of peroxidases in plant defense mechanisms. These results suggest that the shikimate pathway, formerly referred to as secondary metabolism, is differentially activated in response to the pathogen’s presence in the studied carnation varieties.
CONCLUSION
The interaction between host and pathogen in plants is often a complex relationship involving multiple genes expressed at different stages during parasite invasion. Understanding how carnations respond to the presence of the FOX pathogen is a significant challenge for breeders and flower growers. This study reveals variations in the expression levels of various genes related to the response to parasites during the interaction between carnation-resistant and susceptible cells with FOX under in vitro conditions. The construction of gene expression libraries utilized in this study enabled us to identify variations in the expression of genes, including Phenylalanine ammonia-lyase, Anthocyanin 5-O-glucosyltransferase, Beta-1,3-glucanase, Chitinase 4-like glycosyl hydrolase, Peroxidases, and others, which are reported here for the first time as components of the carnation cell’s response mechanism to FOX.
SIGNIFICANCE STATEMENT
The fusariosis produced by FOX is the disease in the carnation that produces more losses around the world. In this job, we demonstrate how resistance to FOX is a process that is regulated by gene groups that are expressed in different forms to produce the resistant or susceptible phenotype to FOX. This finding shows that resistance is more than the relationship between genes and different combinations of resistant genes produce different resistant phenotypes. These results are important in the choice of genes in breeding programs.
ACKNOWLEDGMENT
Our research project was sponsored by the research vice rectory of the “Universidad Militar Nueva Granada” with grant number CIAS-2540.
REFERENCES
- WTO, 2024. Global Trade Outlook and Statistics. World Trade Organization, Geneva, Switzerland, ISBN-978-92-870-7551-2, Pages: 28.
- Wright, C. and G. Madrid, 2007. Contesting ethical trade in Colombia's cut-flower industry: A case of cultural and economic injustice. Cult. Sociol., 1: 255-275.
- Soto-Sedano, J.C., M.J. Clavijo-Ortiz and J.J. Filgueira-Duarte, 2012. Phenotypic evaluation of the resistance in F1 carnation populations to vascular wilt caused by Fusarium oxysporum f.sp. dianthi. Agron. Colomb., 30: 172-178.
- Enespa and S.K. Dwivedi, 2014. Effectiveness of some antagonistic fungi and botanicals against Fusarium solani and Fusarium oxysporum f. sp. lycopersici infecting brinjal and tomato plants. Asian J. Plant Pathol., 8: 18-25.
- Yagi, M., T. Yamamoto, S. Isobe, S. Tabata and H. Hirakawa et al., 2014. Identification of tightly linked SSR markers for flower type in carnation (Dianthus caryophyllus L.). Euphytica, 198: 175-183.
- Li, W., X.L. Wan, J.Y. Yu, K.L. Wang and J. Zhang, 2019. Genome-wide identification, classification, and expression analysis of the Hsf gene family in carnation (Dianthus caryophyllus). Int. J. Mol. Sci., 20.
- Tanase, K., C. Nishitani, H. Hirakawa, S. Isobe, S. Tabata, A. Ohmiya and T. Onozaki, 2012. Transcriptome analysis of carnation (Dianthus caryophyllus L.) based on next-generation sequencing technology. BMC Genomics, 13.
- Gantait, S., T. Subrahmanyeswari and U.R. Sinniah, 2022. Leaf-based induction of protocorm-like bodies, their encapsulation, storage and post-storage germination with genetic fidelity in Mokara Sayan×Ascocenda Wangsa gold. S. Afr. J. Bot., 150: 893-902.
- Filgueira, D.J.J., 2011. Experiences in Carnation (Dianthus cariophyllus) Breeding [In Spanish]. Bogotá: New Granada Military University, Bogotá, Colombia, ISBN: 9789588403410, Pages: 183.
- Filgueira-Duarte, J.J., W.A. Gómez-Corredor and D. Londoño-Serna, 2024. The resistance of carnation (Dianthus caryophyllus L.) to Fusarium oxysporum f.sp. dianthi is a multigene-multivariate phenomenon. Trop. Plant Pathol., 49: 489-501.
- Das, S. and M. Bansal, 2019. Variation of gene expression in plants is influenced by gene architecture and structural properties of promoters. PLoS ONE, 14.
- Ranjan, A., Y. Ichihashi, M. Farhi, K. Zumstein, B. Townsley, R. David-Schwartz and N.R. Sinha, 2014. De novo assembly and characterization of the transcriptome of the parasitic weed dodder identifies genes associated with plant parasitism. Plant Physiol., 166: 1186-1199.
- Widjaja, I., K. Naumann, U. Roth, N. Wolf and D. Mackey et al., 2009. Combining subproteome enrichment and Rubisco depletion enables identification of low abundance proteins differentially regulated during plant defense. Proteomics, 9: 138-147.
- Berger, S., A.K. Sinha and T. Roitsch, 2007. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J. Exp. Bot., 58: 4019-4026.
- Kim, S.C., L. Guo and X. Wang, 2020. Nuclear moonlighting of cytosolic glyceraldehyde-3-phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nat. Commun., 11.
- Chen, Z., X. Shen, J. Wang, J. Wang, Q. Yuan and Y. Yan, 2017. Rational engineering of p-hydroxybenzoate hydroxylase to enable efficient gallic acid synthesis via a novel artificial biosynthetic pathway. Biotechnol. Bioeng., 114: 2571-2580.
- Hyun, M.W., Y.H. Yun, J.Y. Kim and S.H. Kim, 2011. Fungal and plant phenylalanine ammonia-lyase. Mycobiology, 39: 257-265.
- Ardila, H.D., A.M. Torres, S.T. Martínez and B.L. Higuera, 2014. Biochemical and molecular evidence for the role of class III peroxidases in the resistance of carnation (Dianthus caryophyllus L) to Fusarium oxysporum f. sp. dianthi. Physiol. Mol. Plant Pathol., 85: 42-52.
How to Cite this paper?
APA-7 Style
Filgueira-Duarte,
J.J., Londoño-Serna,
D., Hoyos-Carvajal,
L.M. (2025). Gene Expression in Carnation Cells Induced by Fusarium oxysporum f.sp. dianthi. Asian Journal of Plant Pathology, 19(1), 61-71. https://doi.org/10.3923/ajpp.2025.61.71
ACS Style
Filgueira-Duarte,
J.J.; Londoño-Serna,
D.; Hoyos-Carvajal,
L.M. Gene Expression in Carnation Cells Induced by Fusarium oxysporum f.sp. dianthi. Asian J. Plant Pathol. 2025, 19, 61-71. https://doi.org/10.3923/ajpp.2025.61.71
AMA Style
Filgueira-Duarte
JJ, Londoño-Serna
D, Hoyos-Carvajal
LM. Gene Expression in Carnation Cells Induced by Fusarium oxysporum f.sp. dianthi. Asian Journal of Plant Pathology. 2025; 19(1): 61-71. https://doi.org/10.3923/ajpp.2025.61.71
Chicago/Turabian Style
Filgueira-Duarte, Juan, José, Daniela Londoño-Serna, and Liliana María Hoyos-Carvajal.
2025. "Gene Expression in Carnation Cells Induced by Fusarium oxysporum f.sp. dianthi" Asian Journal of Plant Pathology 19, no. 1: 61-71. https://doi.org/10.3923/ajpp.2025.61.71

This work is licensed under a Creative Commons Attribution 4.0 International License.




