Black Hard Rot Disease of Cola nitida and its Fungal Associates
| Received 29 Nov, 2022 |
Accepted 15 Feb, 2023 |
Published 17 May, 2023 |
Background and Objective: Kola nuts botanically calledCola nitida (Vent.) are economic cash crops in Nigeria and have important cultural symbolism in many ethnic groups. A study was conducted to identify the fungal pathogens responsible for kola nuts rot. Materials and Methods: Spoilt kola nuts were collected from Ogbunabali Fruit Garden Market in D-Line, Port Harcourt Local Government Area of Rivers State and morphologically identified. The DNA of the most common fungal isolates, KN-01 was molecularly characterized using Internal Transcribed Spacer 4 and 5 molecular markers. The isolate’s DNA sequence was aligned using the Basic Local Alignment Search Tool for Nucleotide (BLASTN) 2.8.0 version of the National Centre for Biotechnology Information (NCBI) Database. The molecular weight of the DNA of the isolate was 577 Base Pairs. Results: The molecular weight of the DNA of the isolates was 577 base pairs for Aspergillus niger. Based on sequence similarity, it was observed that the C. nitida isolate KN-01 was 99.64% identical to Aspergillus niger isolate with accession number MN474007.1. Conclusion: According to the results, Aspergillus species are among the fungi responsible for the rots in kola nuts. It is well known that Aspergillus niger can infiltrate the lungs and impair immunity. It is hoped that these findings will enhance the knowledge currently available, contributes to the development of efficient disease control strategies for reducing the post-harvest losses brought on by Aspergillus niger and serve as a foundation for further research into the potential mycotoxin effects of ingesting diseased C. nitida.
INTRODUCTION
Kola nut is the fruit of the kola tree plant, belongs to the Malvaceae family and is native to Africa1. Kola nut is an important fruit with economical, medicinal, traditional as well as nutritional values. Economically kola nut is a significant cash crop2 grown by poor rural farmers. The pod husk is used to produce poultry and snail feeds and also to make liquid detergent and organic fertilizer3,4. The kola nut pod husk is widely used for animal feeding5 because of its high nutritive quality.
The use of plants as medicines have received extensive attention6. In ancient times, plants have been in use for medicinal purposes in the treatment of various diseases throughout the world6,7 and to extract the active ingredients from some medicinal plants or plant parts, boiling is needed. Nevertheless, boiling may degrade some bioactive ingredients present in medicinal plants6. Kola extract is an active ingredient in certain pharmaceuticals as it is used as a stimulant and helps to relieve fatigue, treatment of toothaches, depression, constipation, dysentery, weight loss, cough, asthma and migraine headaches5. Traditionally, it is used to settle disputes, used during ceremonies5 related to marriage, child naming ceremonies, installation of chiefs, funerals and sacrifices made to the various gods of African mythology8. These caffeine-containing fruits are used to produce the cola groups beverages such as Coca-Cola, Pepsi cola and many current energy drinks6,9.
Notwithstanding that kola nut contains several components which are beneficiary to man, they also suffer attack from fungi which reduce their economic, medicinal, traditional as well as nutritional value. Therefore, there is a need to identify the fungi responsible for the rots of Cola nitida fruits. The objective of this study was to isolate the fungal organisms associated with Cola spp. rot using traditional and molecular approaches.
MATERIALS AND METHODS
Study area: This research was carried out from 17th January, 2020 to 17th September, 2021 in the Mycology and Plant Pathology Laboratory, Department of Plant Science and Biotechnology, Faculty of Science, University of Port Harcourt, Choba, Nigeria.
Source of plant material: Diseased samples of kola nut fruits, were collected from kola nuts wholesalers in Fruit Market, Port Harcourt, Rivers State, Nigeria.
Isolation of fungi from kola nuts using the blotter method: Fungal pathogens associated with kola nuts were isolated using a standard blotter method modified by Ikechi-Nwogu et al.10. The Petri dishes were lined with 3 layers of sterile filter papers, which were soaked in distilled water. The kola nuts were surface sterilized in a beaker using 70% ethanol, plated and incubated for 7 days at a temperature of 25+2°C. The organism was isolated at the mycology/pathology laboratory of the Department of Plant Science/Biotechnology, Faculty of Science and the DNA was extracted at the Regional Centre for Biotechnology and Biofuel Research Laboratory all in the University of Port Harcourt, Choba, Rivers State, Nigeria.
Purification and sequencing of the PCR products were carried out at the International Institute of Tropical Agriculture (IITA) Ibadan where the most common fungal isolate was coded (KN-01)11.
Morphological and microscopic characterization and identification: The morphological identification of isolates KN-01 was conducted by visually observing the mycelium and comparing their colonies for their diameters, colors, colors of conidia, reverse colors, texture, zonation and sporulation with Snowdon12 pictorial guides. The isolates were later subjected to microscopic analysis for identification using a Celestron electron binocular microscope (Guangzhou, China) at X40. Molecular characterization using the Internal Transcribed Spacer (ITS) marker and identification.
The Genomic DNA of the isolate KN-01 was extracted following the protocol of Quick-DNATM Fungal/Bacterial MiniPrep Kit (Zymo Research Group, California, USA) as described by the manufacturer, with modifications at the Regional Center for Biotechnology and Bioresources (RCBB), University of Port Harcourt, Rivers State, Nigeria. The KN-01 isolate DNA quantity and concentration were measured using the Nanodrop 2000c spectrophotometer (Thermo Fisher Scientific Inc. Wilmington, Delaware, USA). The DNA purity was measured as a ratio of absorbance at 280 nm to that of 260 nanometers. The quality of the DNA of the isolate KN-01 was further quantified using the Agarose gel electrophoresis performed according to the modified method of Saghai-Maroof et al.12. The DNA sample of the KN-01 isolates shipped to the International Institute of Tropical Agriculture (IITA) Bioscience Centre, Ibadan, Nigeria for amplification and sequencing. The primers used to amplify fragments of the nuclear ribosomal DNA (rDNA) of the KN-01 isolates were the Internal Transcribed Spacer 4 (ITS4) with the sequence TCCTCCGCTTATTGATATGS and ITS5 with the sequence GGAAGTAAAAGTCGTAACAAGG. The amplicons were sequenced using the ABI 3500 capillary electrophoresis sequencer. The DNA sequence file was saved in the Bio edit file with an extension .ab1.
Statistical analysis: The sequence was analyzed using the Molecular Evolutionary Genetics Analysis (MEGA) version 7.0.26 software and aligned using the Basic Local Alignment Search Tool for nucleotide (BLASTN) 2.8.0 version of the National Centre for Biotechnology Information (NCBI) Database.
RESULTS
Isolation, morphological and microscopic identification of fungi associated with kola nuts: The result of the fungal isolation was presented in Fig. 1a and the micrograph in Fig. 1b. From the results of the fungal isolation presented in plate 1, an unidentified fungal organism coded KN-01 was isolated and found to be associated with kola nuts. This study investigated the molecular characterization of the fungus associated with kola nuts. Macroscopically, the growth of the KN-01 isolate was white initially but after a few days, it changed to dark black with spores that were visible to ordinary eyes. From the photomicrograph, the isolate was identified as Aspergillus sp.
Molecular characterization using the Internal Transcribed Spacer (ITS) marker and identification. The genomic DNA of the isolates KN-01 of kola nut was successfully extracted. The NanoDrop result showed that the concentration of the DNA of the isolates was 13.7 ng μL–1. While the absorption peak of the 260/280 nm readings was 2.4 and the 260/230 nm readings were 1.2.
The result of the Amplified PCR product generated from the KN-01 isolate was shown in Fig. 2. The amplified DNA showed a band on the gel when observed under UV light. From the result, the ladder used indicated that the KN-01 isolate sequence had over 577 Base Pairs.
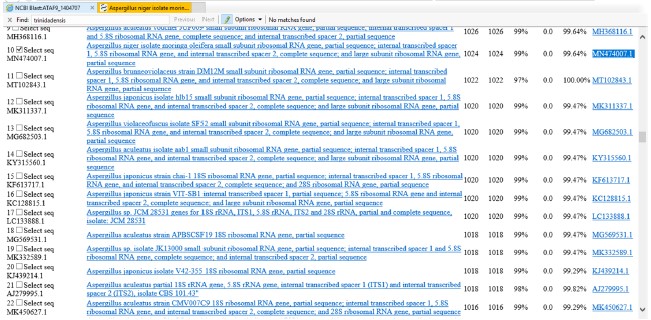
The result of the KN-01 isolate sequence alignment was presented in Fig. 3. The result indicated that the KN-01 isolate sequence aligned with 100 sequences deposited in the composite biological database of the National Centre Biotechnology Information (NCBI). The results showed that the KN-01 isolate sequence was 99.64% identical to Aspergillus niger isolate (purple arrow) with accession number MN474007.1. These findings showed that isolate KN-01 was an Aspergillus spp.

|

|
|
DISCUSSION
Kola nuts are valuable economically, medicinally, traditionally and nutritionally, but numerous fungi attack them during growth, harvest, packing, shipping, storage, marketing and after consumer purchase. According to the research of Moshi et al.13 on the isolation and identification of fungi linked to rotting in kola nuts, these fungi exploited the natural nutrients found in the kola nuts and rendered them inappropriate for human consumption. Accurate identification of pathogens associated with kola nuts rot is essential to the proper development of management strategies.
Before this time, mycologists have relied on the use of phenotypic characters to identify fungal species14, however, the use of traditional methods of identification to identify these fungi, is not as fast and adequate as the molecular method that according to Perincherry et al.15, provides high specificity to distinguish between the species and subspecies of fungi. The molecular techniques used in the identification of fungi in this study led to the successful characterization of a fungus associated with rot in kola nuts. The fungus obtained from this study belongs to the division Ascomycota, class Eurotiomycetes, order Eurotiales and family Trichocomaceae.
In this study, Aspergillus niger was identified as a causal pathogen associated with post-harvest spoilage of kola nuts and characterized by water-soaked patches of rot observed over the infected areas of the nuts. This result was similar to the findings of Adebajo and Popoola16 in their work, identified Aspergillus niger, A. flavus, Penicillium funiculosum, Fusarium moniliforme, A. tamarii, Botryodiplodia theobromae, F. oxysporum and A. orchraceus as the most commonly isolated fungi of kola nuts.
The observation in the study also verified the findings of Oduwaye et al.8. In their study, they isolated and identified A. niger, A. flavus, A. fumigatus, F. oxysporum, L. theobromea and R. stolonifer from soft rot diseased kola nuts. The major cause of post-harvest losses of kola nut is decay caused by the various organisms.
The finding recorded by Oduwaye et al.8 strongly supports the report of this current study as A. niger was also isolated and identified as an organism causing post-harvest losses of kola nut in their report.
According to Navale et al.17, the isolated fungal species belongs to the Aspergillus genera known to have strains that produce toxic metabolites that pose a hazard to consumers.
The findings of Oduwaye et al.8 were in agreement with the fungal isolates of kola nuts reported in this investigation They noted the presence of the same Aspergillus niger, together with other organisms such as Penicillium species, Fusarium species. Aduama-Larbi et al.1 also recorded that Aspergillus specie was the most common genera detected in kola nuts.
High moisture content in kola nuts, is not good for their long preservation as it enhances their susceptibility to fungus infection18. Therefore, it is necessary to control moisture and temperature levels to prevent mold growth and mycotoxin elaboration for the safe storage of kola nuts and also for the safety of consumers1. Ochratoxin A (OTA) in kola nut could eventually be produced as a result of the mold's proliferation. Constant consumption of food or food-related products that are OTA-contaminated could be hazardous to both human and animal health. Therefore, care should be taken during the storage of kola nuts to minimize moisture and fungal contaminations in the market.
CONCLUSION
This study examined the morphological and molecular identification of fungi associated with Cola nitida diseases. From our findings, it is very likely that Aspergillus niger is the causal organism associated with Cola nitida diseases. The common cause of some of the kola nut infections is wounded nuts during harvest and grading processes. Other infections occurred while the kola nuts are in storage. Appropriate pesticides should be developed to reduce the spread of kola nut diseases. Based on these findings, the results iterated that molecular technique remains the best way to identify pathogenic fungi associated with diseases.
SIGNIFICANCE STATEMENT
Based on sequence similarity, this study discovered that isolate KN-01 was 99.64% identical to Aspergillus niger (MN474007.1). Molecular characterization through polymerase chain reaction amplification and sequencing of the Internal Transcribed Spacer (ITS) regions was beneficial in the identification of fungal pathogens causing rots in kola nuts. This study will help the researchers to uncover the critical areas of fungi diseases associated with kola nuts and also enhance disease control which will increase the production yield that many researchers were not able to explore.
REFERENCES
- Aduama-Larbi, M.S., I. Amoako-Attah, W.O. Kumi and S.T. Lowor, 2022. Post-harvest quality assessment of freshly harvested and processed kola nuts [Cola nitida (Vent.) Schott & Endl.] from selected growing regions in Ghana. Cogent Food Agric., 8: 2054565.
- Ekundayo, S.B.O. and E.N. Ugwu, 2015. The semiotic function of the kola nut in Nigeria. Chin. Semiotic Stud., 11: 91-106.
- Asogwa, E.U., A.H. Otuonye, F.C. Mokwunye, K.A. Oluyole, T.C.N. Ndubuaku and E.O. Uwagboe, 2011. Kolanut production, processing and marketing in the South-Eastern States of Nigeria. Afr. J. Plant Sci., 5: 547-551.
- Sofowora, A., E. Ogunbodede and A. Onayade, 2013. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complementary Altern. Med., 10: 210-229.
- Starin, D., 2013. Kola nut: So much more than just a nut. J. R. Soc. Med., 106: 510-512.
- Jamshidi-Kia, F., Z. Lorigooini and H. Amini-Khoei, 2018. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol., 7: 1-7.
- Bukola, F.T., 2018. Conversion of kola nut waste into beneficial products for environmental protection. J. Environ. Sci. Technol., 11: 233-237.
- Oduwaye, O.F., E.C. Omenna and B.A. Ogundeji, 2018. Effect of fungal pathogens on the nutritional qualities of kola nuts (Cola nitida). Acta Sci. Nutr. Health, 2: 5-9.
- Akinnagbe, O.M. and S.F. Ikusika, 2016. Role of household members in kolanut production and marketing in Ekiti State, Nigeria. J. Agric. Extension, 20: 44-58.
- Ikechi-Nwo, C.G., B.A. Odogwu and I.K.G. Uzoka, 2022. Fungal presence in cosmetic facial powder. Asian J. Appl. Sci., 15: 47-52.
- Snowdon, A.L., 1990. A Colour Atlas of Post-Harvest Diseases and Disorders of Fruits and Vegetables: General Introduction and Fruits. CRC Press, Boca Raton, Florida, United States, ISBN-13: 9780723416364, Pages: 302.
- Saghai-Maroof, M.A., K.M. Soliman, R.A. Jorgensen and R.W. Allard, 1984. Ribosomal DNA spacer-length polymorphisms in barly: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA, 81: 8014-8018.
- Moshi, M.J., D.F. Otieno, P.K. Mbabazi and A. Weisheit, 2010. Ethnomedicine of the Kagera Region, North Western Tanzania. Part 2: The medicinal plants used in Katoro Ward, Bukoba District.J. Ethnobiol. Ethnomed. 6: 19.
- Hyde, K.D., K. Abd-Elsalam and L. Cai, 2010. Morphology: Still essential in a molecular world. Mycotaxon, 114: 439-451.
- Perincherry, L., J. Lalak-Kańczugowska and Ł. Stępień, 2019. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins, 11: 664.
- Adebajo, L.O. and O.J. Popoola, 2003. Mycoflora and mycotoxins in kolanuts during storage. Afr. J. Biotechnol., 2: 365-368.
- Navale, V., K.R. Vamkudoth, S. Ajmera and V. Dhuri, 2021. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol. Rep., 8: 1008-1030.
- Ndagi, I., F.D. Babalola, I.U. Mokwunye, C.F. Anagbogu and I.A. Aderolu et al., 2012. Potentials and challenges of kolanut production in Niger State, Nigeria. Int. Scholarly Res. Not., 2012: 492394.
How to Cite this paper?
APA-7 Style
Ikechi-Nwogu,
C.G., Osarolai,
J.O. (2023). Black Hard Rot Disease of Cola nitida and its Fungal Associates. Asian Journal of Plant Pathology, 17(1), 1-6. https://doi.org/10.3923/ajpp.2023.01.06
ACS Style
Ikechi-Nwogu,
C.G.; Osarolai,
J.O. Black Hard Rot Disease of Cola nitida and its Fungal Associates. Asian J. Plant Pathol. 2023, 17, 1-6. https://doi.org/10.3923/ajpp.2023.01.06
AMA Style
Ikechi-Nwogu
CG, Osarolai
JO. Black Hard Rot Disease of Cola nitida and its Fungal Associates. Asian Journal of Plant Pathology. 2023; 17(1): 1-6. https://doi.org/10.3923/ajpp.2023.01.06
Chicago/Turabian Style
Ikechi-Nwogu, Chinyerum, Gloria, and Joseph Osaro Osarolai.
2023. "Black Hard Rot Disease of Cola nitida and its Fungal Associates" Asian Journal of Plant Pathology 17, no. 1: 1-6. https://doi.org/10.3923/ajpp.2023.01.06

This work is licensed under a Creative Commons Attribution 4.0 International License.




